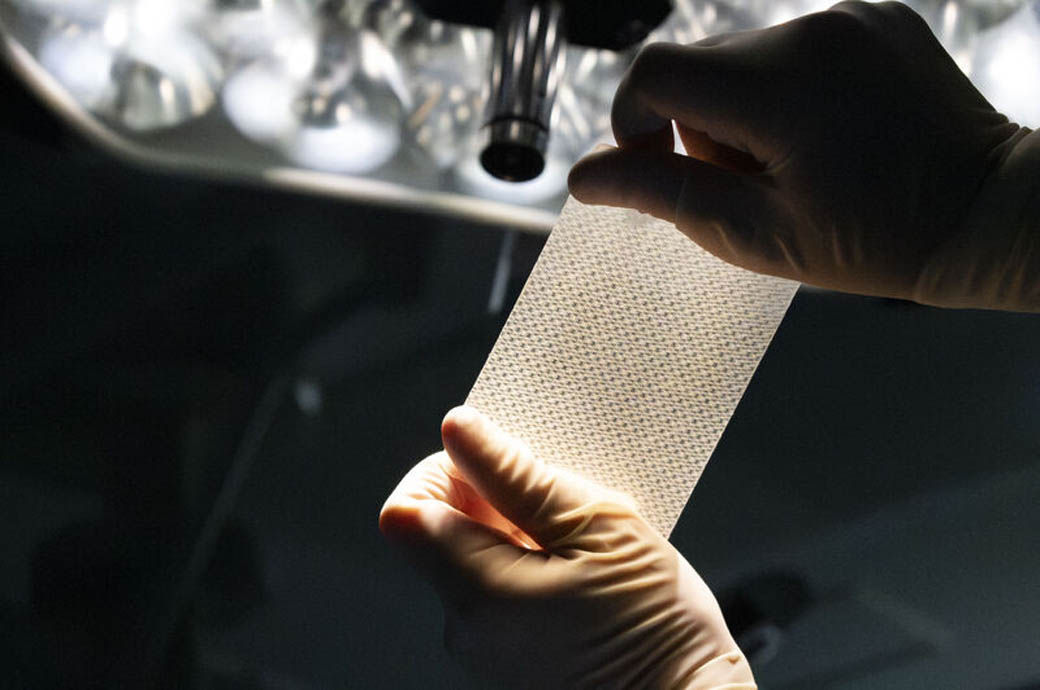
Synfolium is a surgical patch with knitted fabric consisting of both bio-absorbable and non-absorbable yarns coated with cross-linked gelatin. After the patch is surgically implanted into the body, the patient's tissue grows and gradually surrounds the expandable, non-absorbable portion while the bio-absorbable portion degrades. The regenerated tissue has a potential to reduce the risk of inflammatory reactions, foreign body reactions, and cell death that often cause deterioration of the implants.
This new product is to be used in the surgical treatment for patients with congenital heart disease (CHD). Neonates and infants with CHD generally undergo surgeries to correct hemodynamic problems due to septal defects or narrowed blood vessels (stenosis). Over a long period after surgery, a considerable number of them eventually require reinterventions, such as repeat surgery or catheter therapy, due to the deteriorations above.
To address this clinical problem, Shintaro Nemoto of the Osaka Medical and Pharmaceutical University, created an idea of a patch that could accommodate the growth of patients’ bodies by restoring their own tissues. Fukui Tateami, a pioneer in warp-knitting technology, made Nemoto’s idea into a novel concept of an expandable cardiovascular patch that has a special knitted fabric structure to accommodate tissue regeneration and developed a prototype. Teijin, with experience in the healthcare sector through its pharmaceutical and home healthcare businesses, integrated the product design, development and regulatory works into the Synfolium patch. The three parties have been collaborating on development of this product since 2014.
Japan’s ministry of health, labour, and welfare designated the OFT-G1 as a ‘Sakigake’ device in April 2018, providing various incentives to accelerate its approval for clinical use. During the clinical trials of CHD surgery conducted in Japan from 2019 to 2022, there occurred no reoperations and life-threatening events related to the patch. Based on these results, Teijin Medical Technologies submitted a marketing authorisation application in Japan in January 2023.
Going forward, Osaka Medical and Pharmaceutical University, Fukui Tateami, Teijin, and Teijin Medical Technologies will accelerate preparations for the launch of Synfolium in Japan to deliver it to patients as quickly as possible. The consortium will continue to improve the current technology used for Synfolium, and will also expand the product portfolio with the goal of improving the treatment and quality of life of patients with CHD in Japan and worldwide.
"The mission was very challenging, but our development team persevered with strong endurance. This significant milestone was achieved with incredible support from academic societies and regulatory authorities,” said Nemoto. "However, there are still challenges to overcome. We will continue to work together to complete the process of making Synfolium available to cardiac surgeons and their patients with CHD.”
"We hope that Synfolium, which we developed as a strong industry-academia team, will help reduce the burden of re-operation for patients and their families,’’ said Yoshihide Takagi, president of Fukui Tateami. "As we Fukui Tateami celebrate our 80th anniversary next year, we are delighted to contribute to the future of children and to society as a whole through Synfolium.”
"Many surgeons want to reduce repeat procedures as they cause heavy burdens on patients and their families,’’ said Takayuki Nakano, PhD, mission executive and general manager, regenerative medicine and implantable medical device division of Teijin Limited. “We sincerely hope that the Synfolium, an achievement of an industry and academia collaboration with various stakeholders, will solve the issues of patients and their families and help improve their quality of life. In addition, as a manufacturer, we will work to expand the use of this groundbreaking surgical material from Japan to the world, and to develop further medical devices that make use of the concept of Synfolium.”
Synfolium is a significant success made by an industry-academia project, in which academia extracted the concerns of patients in the treatment of CHD, medium and large-sized enterprises brought their technological capabilities and know-how together and their strong cooperative relationships led to industrialisation.
Fibre2Fashion News Desk (RR)

